In 2016, the BSDB introduced the Cheryll Tickle Medal, which is being awarded annually to a mid-career, female scientist for her outstanding achievements in the field of Developmental Biology. 
The BSDB is proud to announce the 2019 awardee Bénédicte Sanson. Due to family commitments, Bénédicte was unable to be at the Spring meeting this year to receive the medal in person, but look out for her interview that will soon appear on the Node.

After a PhD in Paris on the molecular mechanisms of mRNA processing in phage, Bénédicte Sanson switched to Drosophila developmental genetics for a postdoc in Cambridge at the MRC-LMB. During her four-year postdoc with Jean-Paul Vincent (1994-1998), she investigated key aspects of Wingless signalling (the homologue of vertebrate Wnt-1) in development. Through this work, she became aware that the mechanisms underlying cell sorting at compartmental boundaries remained elusive. This fostered a long-standing interest in morphogenesis, which became the focus of her independent research group when awarded a Wellcome Trust Career Development Award in 1998, hosted in the Department of Genetics, Cambridge. Since then, Bénédicte has built up an internationally recognized research group, obtaining a Lectureship in 2009, then a Readership in 2018, in the Department of Physiology, Development and Neuroscience. In 2011 and then in 2017, she was awarded a Wellcome Trust Investigator Award to work on the mechanisms of cell sorting and collective cell movement in vivo.
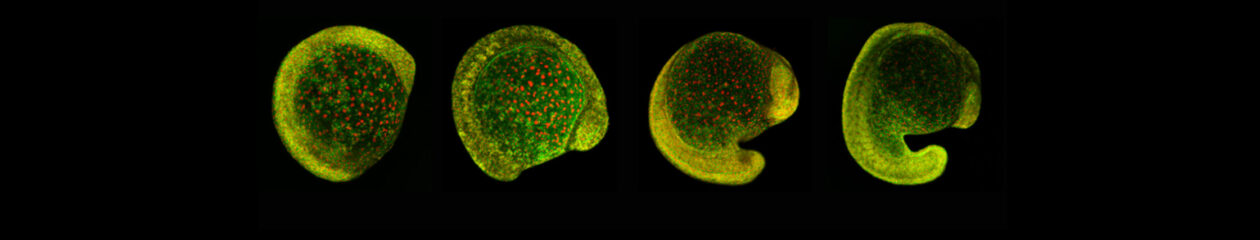
Bénédicte has made key contributions to the field of developmental signaling, including demonstrating that the adhesion and signaling activities of Armadillo (the homologue of vertebrate Beta-catenin) are separable (Sanson et al., 1996) and elucidating novel signaling regulations at the parasegmental organizer in Drosophila embryos (Desbordes and Sanson, 2003; Sanson, 2001; Sanson et al., 1999). More recently, the work of her group has focused on understanding the fundamental processes driving tissue morphogenesis during development. Their work on tissue-scale forces showed that an extrinsic axial force extends the main body axis in Drosophila embryos, acting in parallel to actomyosin-dependent polarized cell intercalations (Butler et al., 2009). Next, they identified the source of this extrinsic force as caused by the invagination of the endoderm at the posterior of the embryo (Lye et al., 2015). Their work on cell sorting demonstrated that actomyosin-based mechanical “barriers” stop cells from invading adjacent compartments, pioneering CALI on GFP in Drosophila embryos to inactivate Myosin II subcellularly (Monier et al., 2010). They further showed that actomyosin-based barriers also order cells during axis extension (Tetley et al., 2016). Recently, the work of her group has shed light onto how actomyosin-driven tension can orientate cell divisions at compartmental boundaries (Scarpa et al., 2018). They also investigated how epithelial folding and actomyosin-enrichment are coupled downstream of Wingless signaling at boundaries (Urbano et al., 2018).
Underlying all of this work is a clear understanding that morphogenesis is dependent on both genetic and physical inputs. As a consequence, Bénédicte’s group often pioneers new methodologies to follow developmental processes quantitatively, and at multiple scales. Their approaches include computational methods to automatically track cell behaviours in real time, for thousands of cells; light sheet imaging (SPIM) to analyse morphogenetic events at the scale of the whole embryo; and laser cuts to probe and manipulate tissue tension. By combining such imaging and computational techniques, the lab continues to investigate how cell intrinsic and extrinsic forces integrate to shape developing tissues. Recently, Bénédicte’s group started developing computational models in collaboration with physicists and mathematicians, to explore the more mechanical aspects of morphogenesis.
In addition to her research contributions, Bénédicte has taught in a range of molecular and developmental genetics courses. Since her appointment in 2009, a significant fraction of her teaching for the University of Cambridge has been for the first year course in Veterinary Anatomy, contributing to the practical element of the course, where the students dissect the different organs and tissues. Other contributions include the active support of postdoctoral careers, both through a previous appointment at the Wellcome Trust to evaluate candidates for early career fellowships, and as Postdoc Committee Chair for her Department.
Selected papers:
Butler, L. C., Blanchard, G. B., Kabla, A. J., Lawrence, N. J., Welchman, D. P., Mahadevan, L., Adams, R. J. and Sanson, B. (2009). Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol 11, 859-864.
Desbordes, S. and Sanson, B. (2003). The glypican Dally-like is required for Hedgehog signalling in the embryonic epidermis of Drosophila. Development 130, 6245-6255.
Lye, C. M., Blanchard, G. B., Naylor, H. W., Muresan, L., Huisken, J., Adams, R. J. and Sanson, B. (2015). Mechanical Coupling between Endoderm Invagination and Axis Extension in Drosophila. PLoS Biol 13, e1002292.
Monier, B., Pelissier-Monier, A., Brand, A. H. and Sanson, B. (2010). An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol 12, 60-65.
Sanson, B. (2001). Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep 2, 1083-1088.
Sanson, B., Alexandre, C., Fascetti, N. and Vincent, J. P. (1999). Engrailed and hedgehog make the range of Wingless asymmetric in Drosophila embryos. Cell 98, 207-216.
Sanson, B., White, P. and Vincent, J. P. (1996). Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383, 627-630.
Scarpa, E., Finet, C., Blanchard, G. B. and Sanson, B. (2018). Actomyosin-Driven Tension at Compartmental Boundaries Orients Cell Division Independently of Cell Geometry In Vivo. Dev Cell 47, 727-740 e726.
Tetley, R. J., Blanchard, G. B., Fletcher, A. G., Adams, R. J. and Sanson, B. (2016). Unipolar distributions of junctional Myosin II identify cell stripe boundaries that drive cell intercalation throughout Drosophila axis extension. Elife 5, e12094.
Urbano, J. M., Naylor, H. W., Scarpa, E., Muresan, L. and Sanson, B. (2018). Suppression of epithelial folding at actomyosin-enriched compartment boundaries downstream of Wingless signalling in Drosophila. Development 145.



 Kate was first introduced to the core questions of developmental biology at the University of Sussex. She then started her research career as a graduate student in Cambridge where she already showed originality of thought and direction with an independent project on the neural development of the earthworm. This interest in understanding how a simple nervous system forms was pursued further supported with a Harkness fellowship in Berkeley, California, where she investigated leech development. On returning to UK, Kate switched to studying the development of the vertebrate nervous system where, over the years, she has made a string of exciting and important discoveries. This work has gained her international recognition in the field of developmental neurobiology and it is this, together with her many contributions to the developmental biology community, that has led to her being awarded the 2019 Waddington medal from the BSDB.
Kate was first introduced to the core questions of developmental biology at the University of Sussex. She then started her research career as a graduate student in Cambridge where she already showed originality of thought and direction with an independent project on the neural development of the earthworm. This interest in understanding how a simple nervous system forms was pursued further supported with a Harkness fellowship in Berkeley, California, where she investigated leech development. On returning to UK, Kate switched to studying the development of the vertebrate nervous system where, over the years, she has made a string of exciting and important discoveries. This work has gained her international recognition in the field of developmental neurobiology and it is this, together with her many contributions to the developmental biology community, that has led to her being awarded the 2019 Waddington medal from the BSDB. Kate Storey is now the head of the Division of Cell & Developmental Biology and Chair of Neural Development, in the School of Life Sciences, at the University of Dundee in Scotland. She investigates cellular and molecular mechanisms regulating neural differentiation in chick and mouse embryos as well as in mouse and human embryonic stem cells. By combining the advantages of each of these experimental systems, Kate has been able to gain substantial insights into the fundamental and conserved processes that regulate vertebrate neurogenesis. Her work has pioneered innovative live imaging approaches for monitoring behaviour and signalling of individual cells within developing tissues. These approaches have led to discovery of a new form of cell sub-division, named apical abscission, as well as providing insights into cell signalling dynamics that underpin asymmetric cell division. Not only is this work an excellent example of what can be learnt from observing cell biology within its normal context in vivo, it also pointed to new mechanism by which signalling is regulated during differentiation. Understanding how the dynamics of neuronal specification and differentiation is controlled during early development is a continued theme in Kate’s work.
Kate Storey is now the head of the Division of Cell & Developmental Biology and Chair of Neural Development, in the School of Life Sciences, at the University of Dundee in Scotland. She investigates cellular and molecular mechanisms regulating neural differentiation in chick and mouse embryos as well as in mouse and human embryonic stem cells. By combining the advantages of each of these experimental systems, Kate has been able to gain substantial insights into the fundamental and conserved processes that regulate vertebrate neurogenesis. Her work has pioneered innovative live imaging approaches for monitoring behaviour and signalling of individual cells within developing tissues. These approaches have led to discovery of a new form of cell sub-division, named apical abscission, as well as providing insights into cell signalling dynamics that underpin asymmetric cell division. Not only is this work an excellent example of what can be learnt from observing cell biology within its normal context in vivo, it also pointed to new mechanism by which signalling is regulated during differentiation. Understanding how the dynamics of neuronal specification and differentiation is controlled during early development is a continued theme in Kate’s work.
