Mariya’s work so far
Upon receiving a 4-year VIB International PhD Program grant, Mariya joined the lab of An Zwijsen in Leuven, Belgium to study the origins of amniotic stem cells and to dissect the unique extraembryonic defects of the Smad5 knock-out mouse embryos. SMAD5 is a downstream effector of BMP signaling, a major pathway involved in many processes in development and cancer. Mariya was fascinated by how entangled the development of embryonic and extraembryonic tissues during early development is, and appreciated the importance of understanding better these neglected parts of the conceptus. She contributed to the finding that Smad5 mutant embryos develop an ectopic primitive streak-like/tumor-like structure in their amnion due to defective signaling (Periera et al., 2012, Development 139(18)), and identified amnion-specific set of marker genes for mouse and human (Dobreva et al., 2012, Stem Cells Int. 987185). The culmination of Mariya’s PhD and postdoc work at Zwijsen’s lab was her most recent paper entitled “Amniotic ectoderm expansion in mouse occurs via distinct modes and requires SMAD5-mediated signalling” (Dobreva et al., 2018, Development 145(15)). This work impressed the judges of the Denis Summberbell Lecture award as a thorough study that sheds light upon both the origin of amnion and the molecular dynamics of its development combining cutting-edge, classical, and original techniques.
After a career brake, Mariya received a 2-year Marie Skłodowska-Curie fellowship and in 2016 moved to the UK to join the lab of Arkhat Abzhanov at Imperial College London. Expanding her research interests towards evolutionary developmental biology, she currently studies the developmental mechanisms underlying the rapid evolution and adaptive radiation of Darwin’s finches from Galapagos islands.
Lecture abstract:
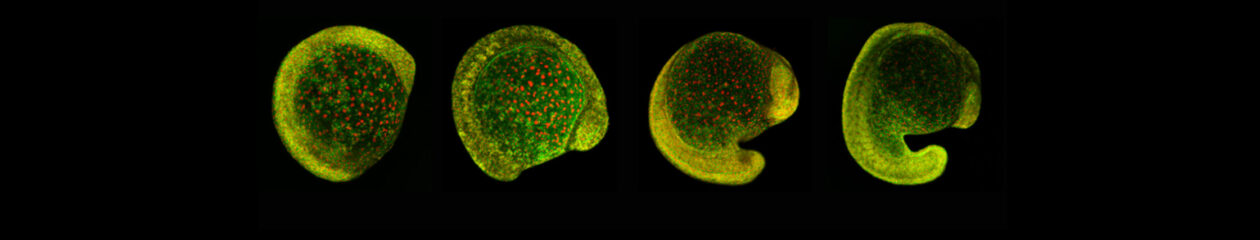
Upon gastrulation, the mammalian conceptus transforms rapidly from a simple bilayer into a multi-layered embryo enveloped by its extraembryonic membranes. The embryonic-extraembryonic junction is a hot spot for dynamic cell rearrangements that drive early morphogenesis. The innermost extraembryonic membrane, the amnion, develops at the embryonic-extraembryonic interphase and gradually encases the developing conceptus. Impaired amnion development causes major embryonic malformations, yet its origin remains ill-defined. Mouse embryos, deficient in the BMP signalling effector SMAD5, show aberrant amnion and ventral folding morphogenesis and delayed closure of the proamniotic canal. I developed a microdissection technique and sequenced the transcriptomes of individual Smad5 mutant amnions isolated before the first visible malformations appear (E7.0-E7.5). I revealed two sets of defective amnions: one with a primitive-streak mesoderm signature and another one with unexpected chorionic ectoderm signature. Tetraploid chimera and immunostaining assays indicated that, in both cases, a deficit in the expansion of amniotic ectoderm results in inclusion of non-amniotic, non-squamous tissues in the amniotic microenvironment. Interestingly, the inclusions can be either of embryonic or of extraembryonic origin. To explain the different types of Smad5 mutant defects and to clarify the origin of mouse amnion, we related our findings to existing clonal analysis of early mouse embryos performed by Kirstie A. Lawson (University of Edinburgh). She traced the fate of single cells labeled before amnion formation. Four clone types contribute to the amniotic ectoderm with distinct growth patterns. Two main clone types were identified, with progenitors in the extreme proximal-anterior epiblast. Their early descendants initiate and expand amniotic ectoderm posteriorly, following the progression of the developing amniochorionic fold. Surprisingly, descendants of cells remaining anteriorly, later expand the amniotic ectoderm from its anterior side. The progenitor regions of all types are close to BMP sources in extraembryonic ectoderm and visceral endoderm. We attribute the two Smad5 mutant defect types to impairment of progenitors of the two main cell populations in amniotic ectoderm, and to compromised cuboidal-to-squamous transition of the anterior amniotic ectoderm. In both cases, SMAD5 is critical for expanding the amniotic ectoderm rapidly into a stretchable squamous sheet to accommodate exocoelom expansion, axial growth and folding morphogenesis.
See article: Dobreva et al., 2018, Development 145(15).